Rethinking Screw Fixation: SupraFusion Augmentation Shows Bone Safety and mechanical stabilty Under Load
SupraFusion® - augmented titanium screws preserve bone integrity, show no adverse tissue response, and support safe polymer degradation and new tissue formation under mechanical loading
SupraFusion® Technology, already known for superior primary mechanical stability [1, 2], has been applied to a new challenge: enhancing titanium screws in load-bearing fixation. While the fundamental science is well-established, the real question we are here addressing is: how does this impact safety, performance, and long-term reliability?.
Study overview
In a 24-month preclinical study using a sheep model, titanium screws were augmented with PLDLLA polymer using SupraFusion® Technology. The screws were implanted in the spine to simulate functional mechanical loading and spinal fixation.
The aim was to assess the biocompatibility of SupraFusion® - augmented titanium screws compared to standard titanium screws in a load-bearing bone environment.
Results
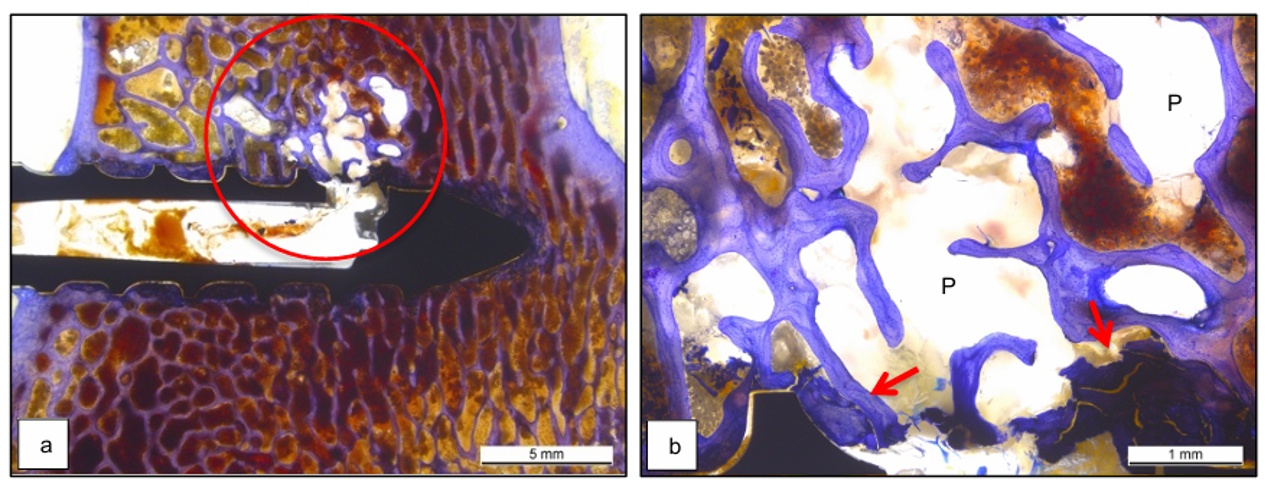
Preservation of the trabecular integrity
The polymer flow of liquefied PLDLLA during augmentation did not damage the trabecular bone structure.
Overview of the extrusion hole and infiltration zone in an augmented titanium screw with absence of isolated trabecular fragments (a). Magnification view of the polymer infiltrating zone (b). Red arrows represent some bone debris due to screw insertion.
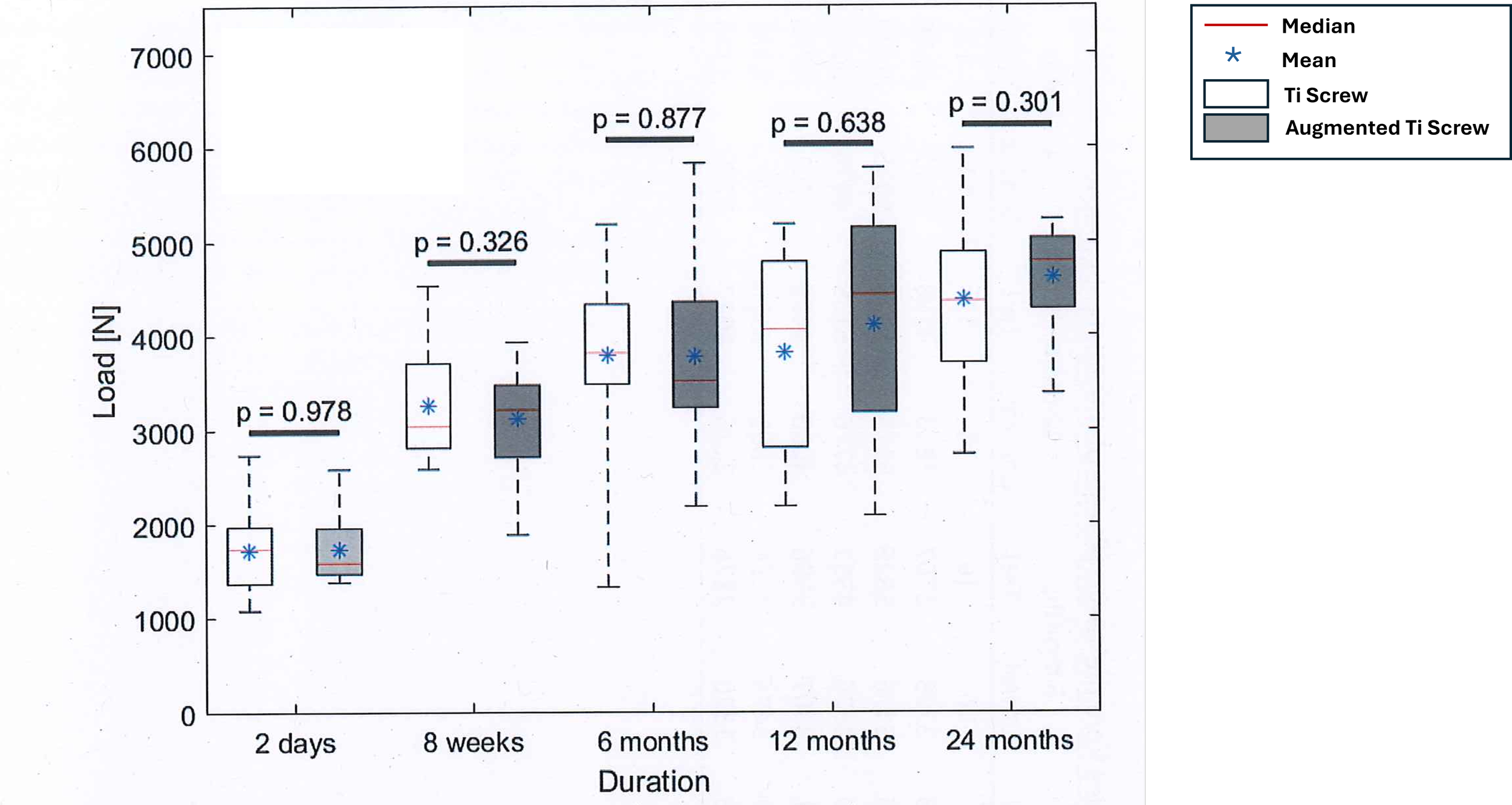
Good mechanical performance
Augmented titanium screw showed overall better pullout load and lower variance as compared to titanium screw, although data were not statistically different.
Comparison of the maximum cyclic pullout load values between augmented titanium screw (grey) and titanium screws (white).
Conclusion
The findings highlight the potential of SupraFusion® Technology for augmenting titanium screws in demanding orthopedic applications – supporting new bone tissue formation, enhancing mechanical stability without compromising bone health.
References
[1] Ferguson S. Bone Welding Mechanical Characterization. An Investigation of the thermal effects and resulting interfacial strength of polymer dowels inserted in bone with ultrasonic welding. Switzerland: M.E. Müller Institute for Biomechanics. University of Bern; 2001 Jul p. 12.
[2] Meyer DC, Mayer J, Weber U, Mueller A, Koch PP, Gerber C. Ultrasonically implanted PLA suture anchors are stable in osteopenic bone. Clin Orthop Relat Res. 2006 Jan;442:143–8.
Note: All findings are from preclinical studies.
No adverse biological reaction
Few PLDLLA implant residuals after 24 months post-implantation with minimal-to-no biological adverse reaction.
Histological picture of the screw hole (S) after 24 months implantation of a Titanium Screw (a) or augmented titanium screw (b). Histological detail at 24 months post-implantation (c) with residual polymer (P) free and associated Giant cells (GC) surrounded by fat (F) and bone marrow cells (C).
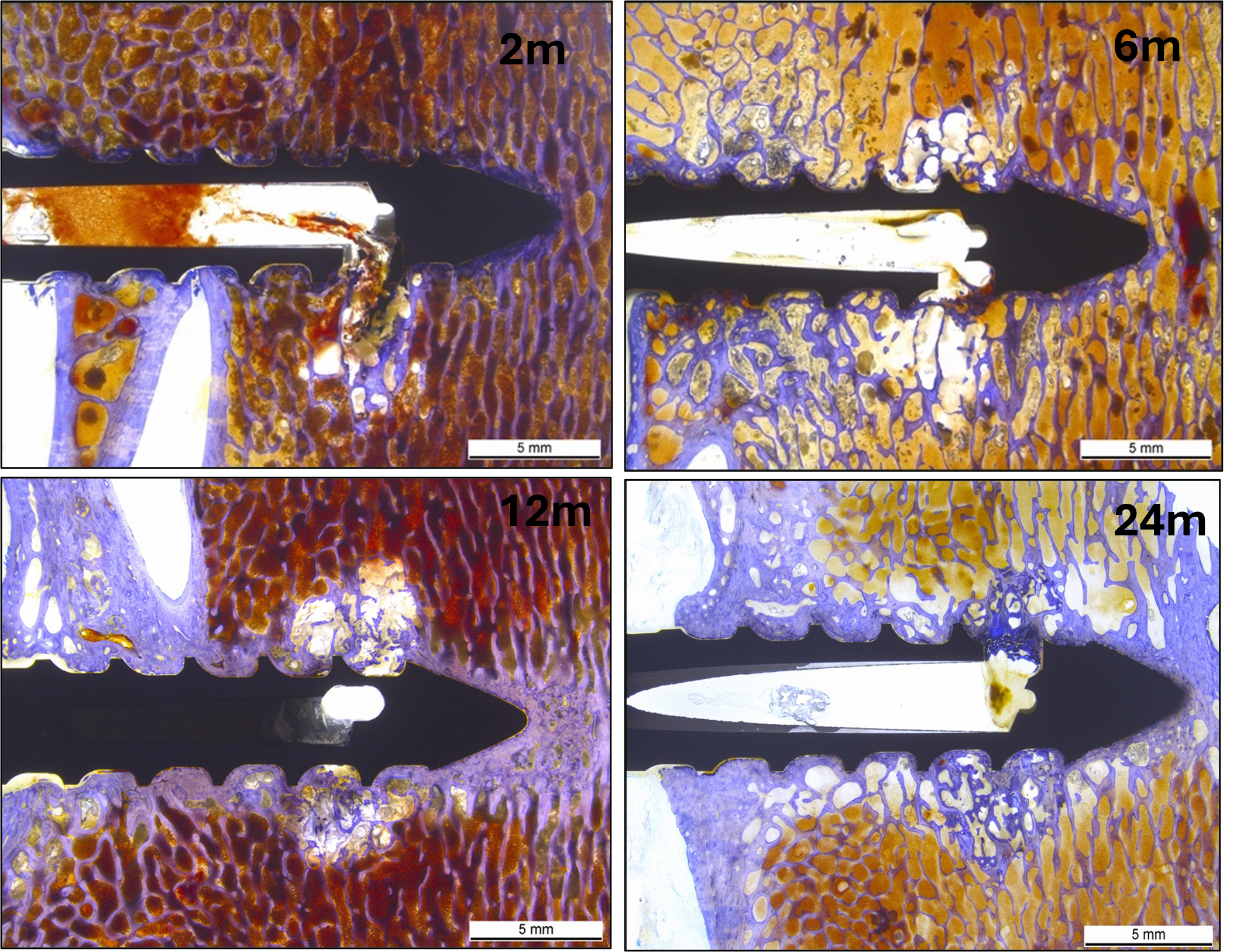
Progressive PLDLLA degradation leading to new bone formation
PLDLLA degraded gradually over 24 months with concurrent bone tissue formation. Histological picture of an augmented Titanium screw with overview of the polymer distribution after 2 months (a), 6 months (b) 12 months (c) or 24 months (d) post-implantation. At 24 months, only traces of polymer residuals were left and replaced by new bone without causing any adverse or inflammatory reaction. One or two extrusion holes were visible depending on the plane of the histological section.